Welcome!
Are you looking for information on patents, trademarks, designs or further market information? Your focus is in chemistry or pharma?
Then you are exactly right here.
Important patent expirations, information on supplementary protection certificates in Europe, expected market entry of generics?
The InPharmation team supports you!
Contact us by mail and discuss with us your information needs.
IP data of pharmaceuticals in Germany
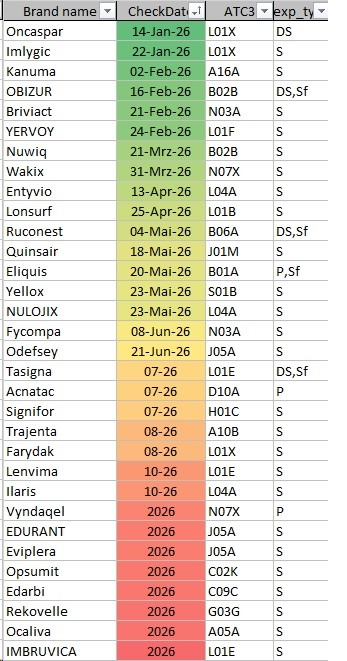
Patent expiries of pharmaceuticals often have great economic importance. In many cases these loses of patent protection are associated with significant revenue losses for the originator as generic competitors push for the market. For other stakeholders in the healthcare system, such as doctors, patients or health insurance, they might bring alternatives to the original product at reduced prices. For manufacturers and developers, the predicted patent expirations are important in order to start in good time with the development of generic medicines and start the approval process.
Intellectual property rights in the chemical and pharmaceutical sector are a particular focus of InPharmation. As an example, the table of expiry data in 2026 are listed. In general, pharmaceuticals are protected from generic competition by several industrial property rights (patents, SPCs, trademarks, designs, data protection). This list represents only a selection of data and indicates a possible generic entry after this date. No guarantee can be given for completeness or correctness of the data
More details are available on request, including patent/SPC expirations in the next 15 years at
Pegaspargase / Talimogen laherparepvec / Sebelipase alfa / Susoctocog alfa / Brivaracetam / Ipilimumab / Simoctocog alfa / Pitolisant / Vedolizumab / Tipiracil/Trifluridin / Conestat alfa / Levofloxacin / Apixaban / Bromfenac / Belatacept / Perampanel / Emtricitabin/Rilpivirin/Tenofoviralafenamid / Nilotinib / Clindamycin/Tretinoin / Pasireotid / Linagliptin / Panobinostat / Lenvatinib / Canakinumab / Tafamidis / Rilpivirin / Emtricitabin/Rilpivirin/Tenofovirdisoproxil / Macitentan / Azilsartan / Follitropin delta / Obeticholsäure / Ibrutinib / Oncaspar / Imlygic / Kanuma / OBIZUR / Briviact / YERVOY / Nuwiq / Wakix / Entyvio / Lonsurf / Ruconest / Quinsair / Eliquis / Yellox / NULOJIX / Fycompa / Odefsey / Tasigna / Acnatac / Signifor / Trajenta / Farydak / Lenvima / Ilaris / Vyndaqel / EDURANT / Eviplera / Opsumit / Edarbi / Rekovelle / Ocaliva / IMBRUVICA
Last update Jan 2024. The data compilation listed here is protected by copyright.(CC BY-NC-SA) For extended use, we kindly ask you to contact us:
INN: International non-proprietary name of the active ingredients of the medicinal product
Generic: Expected entry of generic products (last day of patent or SPC or document protection)
ATC: Anatomical-Therapeutic-Chemical Classification Code according to WHO
IPCM: First IPC Code of Protection Certificate (SPC) as listed in the Register
SPC_EXP: Last day of validity of the supplementary protection certificate
Notes:
D: determining date - Data exclusivity -> market protection
P: determining date - patent expiration
S: determining date - SPC
Sf: SPC requested (additional protection term expected)
S, G: determining date - SPC, generics available
Example of data record:
Aprepitant DE69425161 EP0734381 WO95016679 DE-Pat expiry 13.12.2014 1st EU MA: 11-Nov-03 protection bye: 13.05.2019 ATC-Code: A04AD12 IPC-M: C07D265/32 SPC-expiry: 13.05.2019 decisive date: S =SPC expiry
For expiry dates years 2025 - 2030 please check Patent Expiries 2025-2030
More SPC related data please click here: DE-SPC facts


